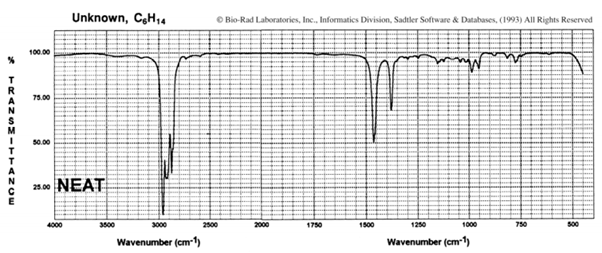
Suggest a possible structure for the hydrocarbon C6H14, which has the infrared spectrum shown here Is there more than one correct structure?
i. Suggest a possible structure for the hydrocarbon C6H14, which has the infrared spectrum shown here: Is there more than one correct structure?
ii. The hydroxylamine I can be oxidized by MnO2 to the amide oxohaemanthidine (II). In dilute solution, the carbonyl absorption band of II occurs at 1702 cm–1 . Explain this observation.
Save your time - order a paper!
Get your paper written from scratch within the tight deadline. Our service is a reliable solution to all your troubles. Place an order on any task and we will take care of it. You won’t have to worry about the quality and deadlines
Order Paper Nowa. 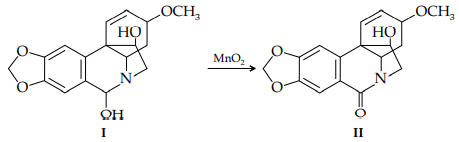
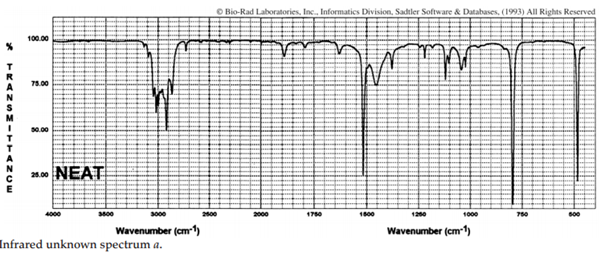
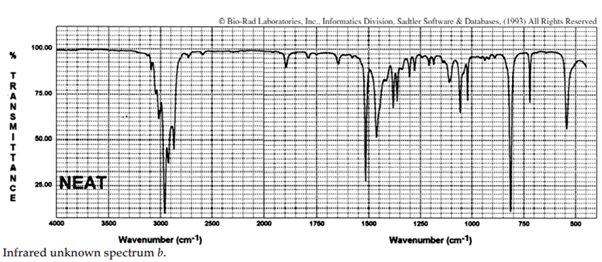
iii. The infrared spectra of the three xylene (dimethylbenzene) isomers, and an additional aromatic hydrocarbon, are given below. Assign the spectra to the isomers and suggest a potential structure for the remaining unknown substance.
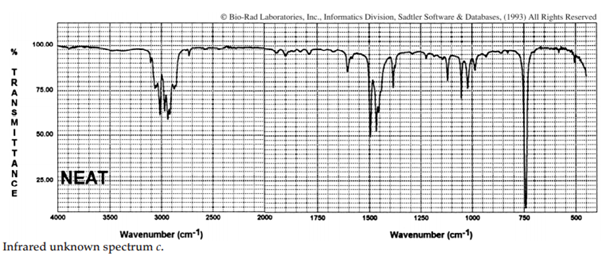
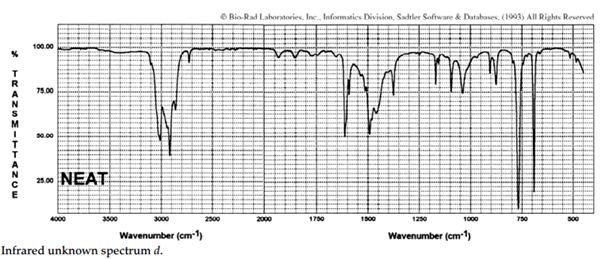
iv. The COH stretching mode of chloroform (CHCl3), which occurs at 3022 cm–1 , is one of the rare exceptions to the 3000-cm–1 rule. What is the rule? Suggest an explanation for this exception.

The post Suggest a possible structure for the hydrocarbon C6H14, which has the infrared spectrum shown here: Is there more than one correct structure? appeared first on Best Custom Essay Writing Services | EssayBureau.com.


