Explain why the 25% NaOH solution is added to the reaction mixture.
i. Explain why the 25% NaOH solution is added to the reaction mixture.
ii. Draw a structure to illustrate the hydrogen bonding that may occur when two polymer molecules of this polyamide are cold – drawn together.
iii. Predict the structure of the polymer that would result in the condensation of the following reactants. These monomers are used to produce the polyamide Nomex, a high-melting material used as an insulator in space shuttles and as the fire-resistant fabric in clothing worn in race cars.
Save your time - order a paper!
Get your paper written from scratch within the tight deadline. Our service is a reliable solution to all your troubles. Place an order on any task and we will take care of it. You won’t have to worry about the quality and deadlines
Order Paper Now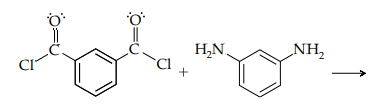
iv. Amides undergo hydrolysis to carboxylic acids on treatment with alkali. Diagram a suitable mechanism for the conversion of benzamide to benzoic acid using sodium hydroxide as the base.

The post Explain why the 25% NaOH solution is added to the reaction mixture. appeared first on Best Custom Essay Writing Services | EssayBureau.com.


