Explain why a single carbonyl band would be expected in the system and why this vibration is located at 1673 cm-1 .
1.Conjugation of the functional group in alkyl isocyanates has little impact on the antisymmetric ![]() stretching vibration located near 2770 cm–1 Explain.
stretching vibration located near 2770 cm–1 Explain.
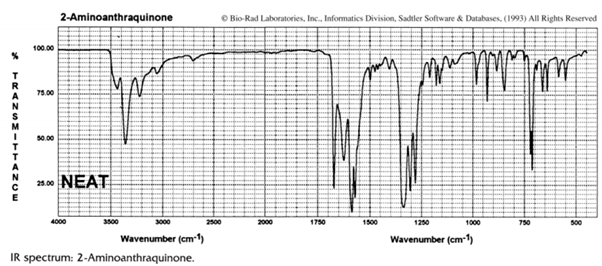
2.In the infrared spectrum of 2-aminoanthraquinone (I) two carbonyl stretching frequencies are observed at 1673.5 and 1625 cm–1 :
Save your time - order a paper!
Get your paper written from scratch within the tight deadline. Our service is a reliable solution to all your troubles. Place an order on any task and we will take care of it. You won’t have to worry about the quality and deadlines
Order Paper Now(a) Assign carbonyl bands in the infrared spectrum to the carbonyl groups in structure I and explain your reasoning
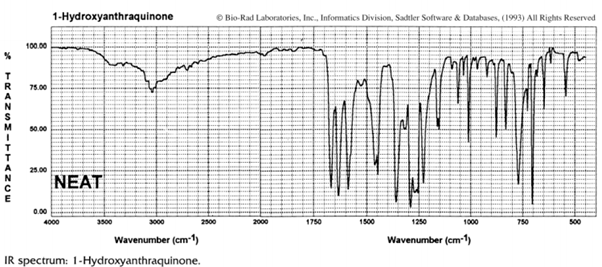
(b) The infrared spectrum of 1-hydroxyanthraquinone (II) also exhibits two carbonyl frequencies, which are located at 1675 and 1637 cm–1 . Assign the carbonyl groups to the related absorption bands. Explain your reasoning.
(c) The spectrum of 2-hydroxyanthraquinone exhibits a single carbonyl stretching frequency near 1673 cm–1 . Explain why a single carbonyl band would be expected in the system and why this vibration is located at 1673 cm–1 .

The post Explain why a single carbonyl band would be expected in the system and why this vibration is located at 1673 cm-1 . appeared first on Best Custom Essay Writing Services | EssayBureau.com.


